CYTO REST 30ml BY NHS
CYTO REST 30ml BY NHS
CYTO-REST Transdermal Cream
****Compounded products- sales final, no returns, no exchanges****
Cyto-Rest cream is supplied in a 20ml clicker to allow exact dosing of cream.
Cyto-Rest cream should be applied to freshly washed and dried skin and rubbed in until completely absorbed.
20 clicks per dose, 20 doses per clicker.
Cyto-Rest cream the following in a pharmacy quality transdermal base cream:
· Maritime Pine Bark Extract Powder, Pinus Pinaster, Standardised active: 95% Proanthocyanidins (OPC). 20 mg per dose
· Perilla Frutescens 10:1 Extract 40 mg per dose
· Benfotiamine 20mg per dose
· apigenin 5mg per dose
· Baicalin (Skullcap Root 10:1 Extract Powder. Scutellaria Baicalensis.) 20mg per dose
· Tulsi 25mg per dose
· vitamin A Retinyl Palmitate 3,000 iu per dose
· vitamin D3 Cholecalciferol 2,000 iu per dose
· vitamin K2 Menaquinone 7 100mg per dose
· vitamin E Tocotrienols 5mg per dose
· Riboflavin (vitamin B2) 20mg
· CNS / ANS Biopeptide regulators
Pharmacy quality transdermal base
Our Transdermal case is an organic gel made from lecithin. It has been formulated to have cosmetic properties, being non-greasy and having improved skin penetration of incorporated active ingredients. After application to the skin, it rapidly disappears leaving no residue. It has been widely accepted as a superior vehicle for delivering drugs across the skin barrier where high local concentrations of drug are obtained from small applied doses. It also allows drug to penetrate the skin reaching the general circulation when desired, making the topical route possible.
Maritime Pine Bark Reduces Inflammation
Cytokines are a group of pro-inflammatory molecules that are released when you get a cold or flu, suffer from a fall or injury (or train too hard in the gym), or if you’re struggling with a chronic health condition. Research shows that pine bark extract is capable of reducing pro-inflammatory cytokines IL-1 and IL-6, responsible for many of the adverse effects of chronic inflammation.
Pine bark extract can make a great addition to your nutritional arsenal for its potent antioxidant support, as well as its added support for blood flow, blood sugar, inflammation, immunity, brain function and skin support.
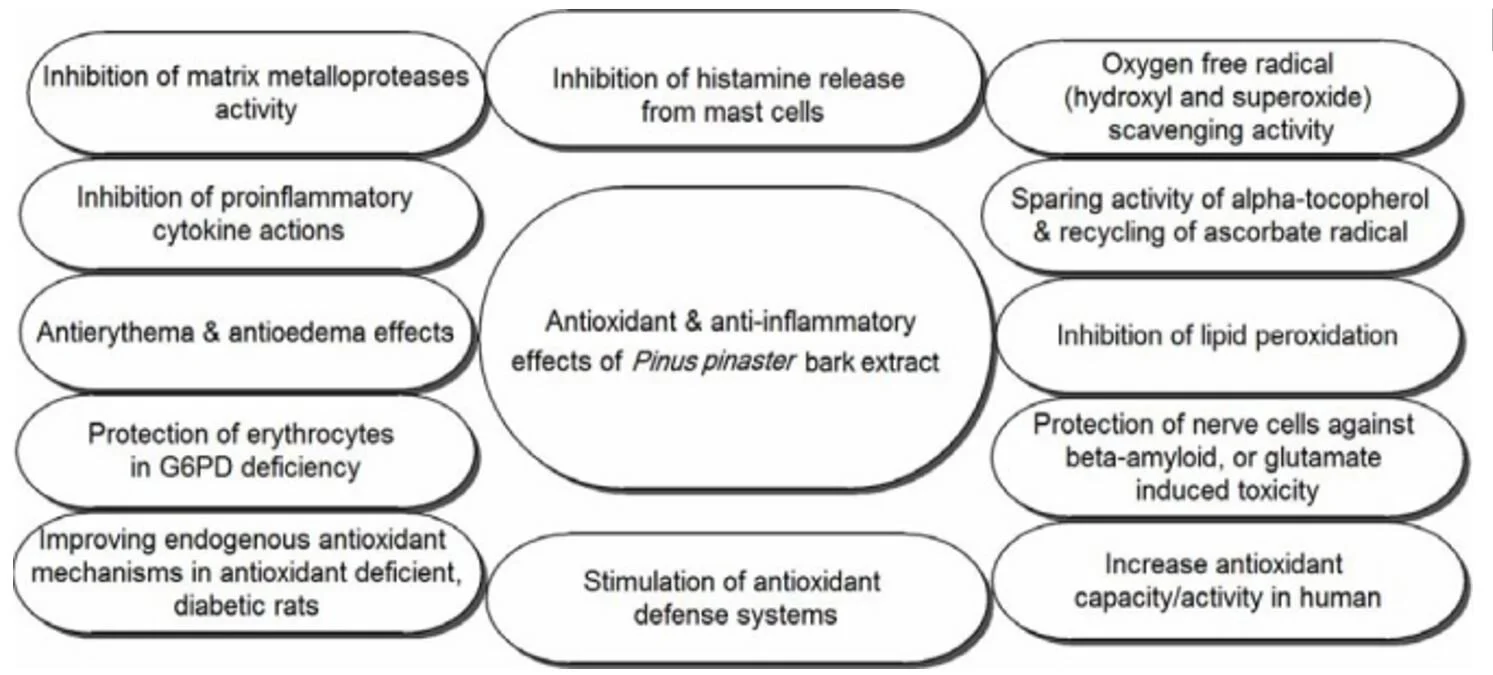
Antioxidant and free radical scavenging activities
Inactivation of the superoxide and hydroxyl radical, and inhibition of singlet oxygen formation are two important beneficial effects of PBE. PBE was reported to exert protective effects against lipid peroxidation, thiobarbituric acid reactive products generation, and oxidative hemolysis induced by peroxide hydrogen. Furthermore, it prevents accumulation of oxidatively damaged proteins and may reduce the risk of several neurodegenerative diseases such as Parkinson's, Alzheimer's, and Huntington's diseases. In vitro studies indicated that PBE inhibited peroxidation of low-density lipoprotein cholesterol (LDL), lipid peroxida-tion in phospholipid liposomes, lipid peroxidation caused by t-butylhydroperoxide, and UVB-induced lipid peroxidation in cells.
PBE exhibited a concentration-dependent inhibition of oxidative burst triggered by zymosan in J774 murine macrophage and LDL oxidation. J774 is a murine macrophages cell line established from a tumor which arose in a female BALB/c mouse. Its growth is inhibited by dextran sulfate, purified protein derivative, and bacterial lipopolysaccharides. J774 cells have been used for numerous biological and biochemical investigations aimed at under-standing the physiology of monocytes-macrophages. PBE also minimized the stand cleavage (measured by agarose gel electrophoresis) in pBR322 plasmid DNA caused by hydroxyl radicals produced by iron/ascorbic acid. Plasmid vector pBR322, a well-established multipurpose cloning vector in laboratories worldwide, is designed and constructed for the efficient cloning and selection of recombinant DNA molecules in Escherichia coli. PBE showed synergetic antioxidant effects with vitamin C and E, and lutein for prevention of lipid peroxidation in liposome and porcine retinal homogenate, respectively. The antioxidant and anti-inflammatory effects of PBE have been proven by in vitro and in vivo studies and clinical trials summarized in.
Antioxidant and anti-inflammatory effects of Pinus pinasterbark extract
The putative putative antioxidant activity of PBE has been investigated against CCl4-induced hepatic oxidative damage in Sprague-Dawley rats. A single oral dose of CCl4 (1.25 mL/kg) produced significantly increased levels of serum aminotransferase and alanine aminotransferase activities, increased malondialdehyde concentration, reduced glutathione content, and decreased catalase, superoxide dismutase and glutathione -S-transferase activities in the hepatic tissues. Results of this study showed that administration of PBE (10 or 20 mg/kg) improved CCl4-induced hepatic injury, as evidenced by the decline of serum aminotransferase and alanine aminotransferase activities in a dose dependent manner, reduction of malondialdehyde concentration, and enhancement of glutathione levels and catalase, superoxide dismutase and glutathione-S-transferase activities in hepatic tissues, indicating that administration of PBE efficiently prevents the CCl4-induced oxidative damage in rats. In another study, after administration of PBE, the glutathione (GSH) and GSH-disulphide reductase (GSSG-R) ratio, the activities of endogenous antioxidant enzymes (SOD, CAT, GSH-Px, GSSG-R), and the activity of γ-glutamyl transpeptidase and enzyme in the pathway of glutathione synthesis were increased in streptozotocin-induced diabetic rats. Moreover, it was reported that the consumption of PBE alone or in combination with beta-carotene may lead to an increase in activity of GSSG-R. Decreased retinal γ-GT activity in diabetic rats and elevated activity of superoxide dismutase in diabetic retina due to the oxidative stress were normalized by PBE and beta-carotene combination.
Polyphenols containing PBE were found to have scavenging activity against reactive oxygen and nitrogen species. Decreased nitrogen monoxide (NO) generation due to the scavenging activity against reactive oxygen species and NO, inhibition of iNOS activity, and inhibition of iNOS-mRNA expression were noticed. PBE was shown to have scavenging activities against reactive oxygen species in macrophages and blocks the activation of NF-kappaB and activator protein-1, and inhibits the expression of pro-inflammatory cytokine IL-1, vascular cell adhesion molecule-1 and intracellular adhesion molecule-1.It has also been reported that PBE inhibited TNF-α secretion, nuclear factor (NF)-kappaB-dependent gene expression, and MMP-9 release from lipopolysaccharide-stimulated monocytes (mouse leukemic macrophage cell line, RAW 264.7).
Anti-inflammatory effects
Several in vitro studies have revealed that PBE has anti-inflammatory effects and inhibits the initiation of inflammation by preventing the release of pro-inflammatory mediators regulated by oxidative stress. PBE inhibits the pro-inflammatory mediators in keratinocytes (epidermal cells), leukocytes, and endothelial cells. Furthermore, an in vitro study has shown that PBE and its metabolites inhibit the release of tissue destroying enzymes (matrix metalloprotein-ases) collagenase, elastase, and gelatinase from inflammatory cells. It has also reported that after oral intake of PBE, the enzymatic activity of Cyclo-oxygenase (COX-1 and COX-2) in serum samples of human volunteers was inhibited. Cyclo-oxygenase is responsible for formation of biological mediators, such as prostaglandins, prosta-cyclin, and thromboxane. Pharmacological Inhibition of this enzyme can provide relief from the symptoms of inflammation and pain.
One of the major pro-oxidant challenge, exposure to UV radiation, may lead to the expression of many pro-inflammatory genes including tumor necrosis factor-α (TNF-α), IL-1 α, IL-1β, IL-8, and IL-6 (all these cytokines contain nuclear factor-kappaB binding sites in the 5 flanking region of their encoding gene). Application of PBE topically could be used for significant and dose-dependent protection from solar-simulated UV radiation (SSUV)-induced acute inflammation, photo-carcinogenesis, and immune-suppression when applied after sunburn and daily irradiation. In one study, 21 Caucasian volunteers received PBE (1.1 mg/kg body weight) orally. As a result, the protective effect of PBE against UV-light induced skin damages has been proven. It was demonstrated that PBE suppressed the expression of pro-inflammatory cytokines and decreased the expression of mast cell related tryptase and stem cell factor. The inhibition of NF-kappaB activation in lipopolysaccharide-stimulated monocytes is one of the reasons for anti-inflammatory effects of PBE. PBE inhibited iNOS and iNOS-mRNA expression in murine macrophages, activated by the bacterial wall components lipopolysaccharide and interferon (INF-γ).
In the most industrialized countries, osteoarthritis of the main joints (severe bone and joint abnormalities) is a diffuse social problem affecting the quality of human lives. Among the herbal antioxidative supplement, PBE has beneficial effects on symptoms of osteoarthritis. A double-blind, placebo-controlled study reported that PBE lowered pain and stiffness and improved symptoms of knee osteoarthritis (flexibility of osteoarthritic joints).
Attention deficit hyperactivity disorder (ADHD)
Polyphenols rich extract like PBE reduces hyperactivity of children, catecholamine excretion, and oxidative stress. A randomized, double-blind, placebo controlled study on ADHD children indicated that the treatment with PBE caused decrease of dopamine and trend of adrenaline and noradrenaline decrease, and increased GSH/GSSG ratio. In another randomized, double-blind, placebo-controlled study, the influence of administered PBE or placebo on the level of reduced GSH and oxidized GSSG glutathione in children suffering from ADHD (the neuro-developmental disorder with impulsivity, distractibility and hyperactivity) and on total antioxidant status were investigated. As a result, significant decrease in GSSG and increase in GSH levels, as well as improvement of GSH/GSSG ratio in comparison with placebo group were reported. Moreover, PBE improved the antioxidant status of ADHD children. The effect of polyphenolic extract of pine bark was investigated on the level of oxidized purines represented by 8-oxo-7, 8-dihydroguanine (8-oxoG) and on the total antioxidant status in children with attention deficit/hyperactivity disorder. They found that PBE protected DNA against oxidation, normalized total antioxidant status and improved attention of ADHD children.
Antimicrobial and antiviral activity
PBE rich in procyanidins inhibited not only the binding of human immunodeficiency virus type-1 (HIV-1) to host cells but also inhibited HIV viral replication and T-cell interaction in cell culture experiments. PBE was found to induce expression of an intracellular antioxidant protein and manganese superoxide dismutase, and inhibition of phosphorylation of the ribosomal S6 protein. It seems that these biochemical alterations induced by PBE play an important role in its antiviral effects. PBE is a promising agent for inhibition of encephalomyocarditis viral replication, prevention of development of viral myocarditis, and improvement of inflammation and myocardial necrosis. It was reported that PBE (100 mg/kg) had beneficial effects on viral myocarditis by inhibition of viral replication and by suppression of pro-inflammatory cytokines, genes related to cardiac remodeling, and mast cell-related genes in the heart muscle of mice (gene expressions of tumor necrosis factor, type-1 procollagen, stem cell factor, and mast cell tryptase).
PBE was shown to have antimicrobial activity against various pathogenic prokaryotes (gram negative and positive bacteria), and eukaryotes (fungi and yeasts). For instance, growth and adherence of Helicobacter pylori (the gram negative, microaerophilic bacterium) to mucosal cells of the stomach were inhibited by PBE, in vitro. In another study, antimicrobial effects of PBE against 23 pathogenic microorganisms (e.g., Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeroginosa, Bacillus cereus, Enterococcus faecalis, Candida albicans, Aspergillus oryzae, and Salmonella sp.) have been investigated. Consequently, PBE inhibited the growth of all tested microorganisms in minimum inhibitory dosages (MID) ranging from 20 to 250 μg/mL.
PBE rich in polyphenolic compounds has been shown to cause endothelium-dependent vasorelaxation and decrease the amount of circulating inflammatory substances in the blood stream. Intake of PBE is useful in order to reduce the risk of heart disease and is effective in the treatment of chronic venous insufficiency and retinal micro-hemorrhages venous disorders. PBE, is an antioxidant with low acute and chronic toxicity, can be used in order to ameliorate excessive oxidative stress in several cell systems by doubling the intracellular synthesis of anti-oxidative enzymes and by acting as a potent scavenger of free radicals. Furthermore, PBE plays an important role in the regeneration and protection of vitamin C and E. Anti-inflammatory and antioxidant activities of PBE have been demonstrated in vitro and in vivo. PBE also protects the nerve cells against beta-amyloid, or glutamate induced toxicity and the erythrocytes in G6PD deficiency. Protection against UV-radiation-induced erythema, anti-inflammatory effects in asthma patients, and reduction of attention-deficit disorder and ADHD symptoms in children have been reported in clinical studies following oral intake of PBE. Immunomodulation has been observed in both animal models and patients with Lupus erythematosus. Reduction of smoke-enhanced thromboxane B formation and platelet aggregation in response to cigarette smoking has also been reported. PBE inhibits angiotension-converting enzyme which is associated with a mild antihypertensive effect. PBE relieves menstrual abdominal pain which is attributed to the antispasmodic of some phenolic acids on smooth muscle. Results from pharmacological and clinical studies have demonstrated that PBE can be used as a source of natural antioxidants in the food and pharmaceutical industries.
Perilla Frutescens
Perilla frutescens (L.) Britt. (PF) is an annual herbal medicinal, aromatic, functional food, and ornamental plant that belongs to the mint family, Lamiaceae. The origin of perilla traces back to East Asian countries (China, Japan, Korea, Taiwan, Vietnam, and India), where it has been used as a valuable source of culinary and traditional medicinal uses. The leaves, seeds, and stems of P. frutescens are used for various therapeutic applications in folk medicine. In the absence of a comprehensive review regarding all aspects of perilla, this review aims to present an overview pertaining to the botanical drug, ethnobotany, phytochemistry, and biological activity. It was found that the taxonomic classification of perilla species is quite confused, and the number of species is vague. Perilla has traditionally been prescribed to treat depression-related disease, anxiety, asthma, chest stuffiness, vomiting, coughs, colds, flus, phlegm, tumors, allergies, intoxication, fever, headache, stuffy nose, constipation, abdominal pain, and indigestion, and acts as an analgesic, anti-abortive agent, and a sedative. Until now, 271 natural molecules have been identified in perilla organs including phenolic acids, flavonoids, essential oils, triterpenes, carotenoids, phytosterols, fatty acids, tocopherols, and policosanols. In addition to solvent extracts, these individual compounds (rosmarinic acid, perillaldehyde, luteolin, apigenin, tormentic acid, and isoegomaketone) have attracted researchers’ interest for its pharmacological properties. Perilla showed various biological activities such as antioxidant, antimicrobial, anti-allergic, antidepressant, anti-inflammatory, anticancer, and neuroprotection effects. Although the results are promising in preclinical studies (in vitro and in vivo), clinical studies are insufficient; therefore, further study needs to be done to validate its therapeutic effects and to ensure its safety and efficacy.
Antioxidant Activity
Epidemiological, clinical, and nutritional studies show that consumption of so-called functional foods and nutraceuticals may be associated with a lowered risk of cancers, cardiovascular diseases, and metabolic disorders. These benefits are often attributed to the high antioxidant capacity of the drug, and especially to the content of phenolic acids, flavonoids, and carotenoids. It has been reported that extracts from perilla seeds and leaves exhibit concentration-dependent antioxidant activity, based on the 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) radical assay, and 2,2′-azino-bis(3-ethylbenzothiazoline-6 sulphonic acid) (ABTS) radical cation assay. Isolated rosmarinic acid (RA) and luteolin from the fruit of P. frutescens var. acuta showed significant DPPH scavenging capacity with half-maximal inhibitory concentration (IC50) values of 8.61 and 7.50 µM, respectively. Similarly, among five phenolic compounds, RA and rosmarinic acid-3-O-glucoside were the dominant phenolic antioxidants with strong activity from cold-pressed perilla var. arguta seed flour studied by Zhou et al. RA isolated by these authors from perilla leaf exhibited DPPH radical scavenging activity of 88.3 ± 0.7% at a concentration of 10 μg/mL with a drug concentration eliciting 50% of the maximum stimulation (SC50) value of 5.5 ± 0.2 μg/mL. Tian et al. proved that the antioxidant activity of perilla essential oil may depend on the location of cultivation. Extracts of drugs harvested from different regions exhibited varying degrees of scavenging ability at 10 mg/mL concentrations with an inhibition percentage of 94.80 ± 0.03%. The 80% methanol extract of perilla seeds exhibited a strong antioxidant property . In vivo, the protective activity of RA from P. frutescens leaf (PFL) was demonstrated on Lipopolysaccharide (LPS)-induced liver injury of d-GalN-sensitized mice owing to the scavenging or reducing activities of superoxide or peroxynitrite rather than to inhibition of tumor necrosis factor (TNF)-α production.
The roles of the flavonoid luteolin from the perilla seeds seems to provide significant antioxidant activity for drugs and extracts. This compound significantly reversed hydrogen peroxide-induced cytotoxicity in primary cultured cortical neurons. Whereas, luteolin markedly attenuated the reactive oxygen species (ROS) production, and prevented the decrease in activities of mitochondria, catalase, and glutathione in ROS-insulted primary neurons, for preventing neurodegenerative diseases. In another study, luteolin inhibited the peroxidation of linoleic acid catalyzed using soybean lipoxygenase-1 with an IC50 of 5.0 M (1.43 μg/mL) noncompetitively.
The monoterpene perillaldehyde was shown to be a potent thioredoxin inducer as it activates the Nrf2-Keap1 system . It seems that the antioxidant activity of perilla may vary among different accessions. As part of an in vitro study in a human subjects, purple perilla leaves showed a higher antioxidant activity, and prevented the oxidation of low-density lipoprotein (LDL) than the green leaves . Another study revealed that 2′,3′-dihydroxy-4′,6′-dimethoxychalcone (DDC) found in green perilla leaves enhanced cellular resistance to oxidative damage through activation of the Nrf2-antioxidant response element (ARE) pathway.
Antibacterial and Antifungal Activity
Recently, the demand for natural compounds from plant extracts as effective antibacterial agents against a wide range of bacteria is definitely growing to control human infection and for the preservation of food . Perilla seed extract rich in polyphenols was examined for its antibacterial activity against oral cariogenic Streptococci and periodontopathic Porphyromonas gingivalis. The ethyl acetate extracts exhibit strong antibacterial activity against oral Streptococci and various strains of P. gingivalis. On the other hand, the ethanolic extract of defatted perilla seed weakly inhibited the growth of oral pathogenic bacterial strains. Among the polyphenols, luteolin showed marked antibacterial activity against the oral bacteria tested . Additionally, the antibacterial activity of the essential oil from perilla leaves on Gram-positive and Gram-negative bacteria was studied, and the results showed the effectiveness of this essential oil to inhibit the growth of the tested bacteria. The minimum inhibitory concentration (MIC) on Staphylococcus aureus and Escherichia coli were 500 μg/mL and 1250 μg/mL. respectively. The most abundant terpene-type compound, perillaldehyde, moderately inhibits a broad range of both bacteria in the range of 125–1000 pg/mL. This compound was also particularly active against filament fungi, with MIC values for M. mucedo and P. chrysogenum already at a 62.5 pg/mL concentration. Kim and Choi determined the antibacterial activity of the leaf ethanol extracts of PF var. acuta against S. aureus and Pseudomonas aeruginosa and detected that the population of P. aeruginosa decreased from 6.660 to 4.060 log CFU/mL, and that of S. aureus from 7.535 to 4.865 log CFU/mL, as well as to 2.600 log CFU/mL via extraction with ethyl acetate.
The fungicidal effects of perilla EO were described against Trichophyton mentagrophytes, and they dose-dependently decreased the production of α-toxin, enterotoxins A and B, and toxic shock syndrome toxin 1 (TSST-1) in both methicillin-sensitive S. aureus and methicillin-resistant S. aureus. The antifungal activity of perilla EO distilled from aerial parts of the plant was also tested against phytopathogenic fungi and its activity was demonstrated in the cases of Aspergillus flavus, Aspergillus oryzae, Aspergillus niger, Rhizopus oryzae, and Alternaria alternate.
Anti-Allergic Effect
Studies show that water extracts of PF may inhibit allergic reactions in vivo and in vitro. PF extracts (0.05 to 1 g/kg) greatly inhibited systemic allergic reactions activated by anti-DNP IgE in rats in a dose-dependent manner. Similarly, the water extract of PFL has been shown to have a positive result against atopic dermatitis in an animal model. The anti-allergic effects of PFL on 2,4-dinitrofluorobenzene (DNFB)-induced atopic dermatitis in C57BL/6 mice was evaluated by Heo et al. and the results revealed that an aqueous extract (100 μg/mL) of PFL could significantly inhibit DNFB-induced atopic inflammation by alleviating the expression of MMP-9 and IL-31, as well as augmenting T-bet activity. In another experiment, water extract from PFL significantly suppressed the PCA-reaction, using mice ear-passive cutaneous anaphylaxis (PCA)-reaction, and the authors concluded the role of rosmarinic acid. Application of an ethanol extract from PF, rather than the aqueous extract, suppressed the allergen-specific Th2 responses. Furthermore, airway inflammation and hyperreactivity in an ovoalbumin-sensitized murine model of asthma were alleviated. Based on this, Chen et al. suggested perilla as a potential phytotherapeutic tool for immunomodulation.
Besides using crude extracts, individual compounds as a potential biologically active agent against allergies have also been studied. A novel glycoprotein fraction from the hot water extract of perilla was used and it was found that it moderately inhibited mast cell degranulation and the activities of hyaluronidase (IC50 = 0.42 mg/mL) in a dose-dependent manner . Furthermore, daily oral supplementation with RA (1.5 mg/mouse, orally) from perilla significantly prevented the increase in the numbers of eosinophils in bronchoalveolar lavage fluids and in those around murine airways. Likewise, the expression of IL-4 and IL-5, and eotaxin in the lungs of sensitized mice, together with allergen-specific IgG1, were also inhibited. Due to these findings, the authors revealed RA as an effective intervention against allergic asthma . In other study, perilla extracts enriched with RA could inhibit seasonal allergic rhinoconjunctivitis in humans at least partly via inhibition of polymorphonuclear leukocytes (PMNL) infiltration into the nostrils . The use of a diet supplemented with perilla oil might be effective on asthmatic allergy via decreasing serum lipids and ovalbumin-specific IgG1 and IgA levels in mice .
Anti-Depressant Activity
Numerous studies focusing on the extracts and/or purified compounds of P. frutescens displayed antidepressant-like effects. Phenolic-type constituents of perilla leaf, such as apigenin, at intraperitoneal doses of 12.5 and 25 mg/kg , RA (2 mg/kg, i.p.) and caffeic acid (4 mg/kg, i.p.) each led to a considerable reduction of the duration of immobility in the forced swimming test. These compounds are also supposed to inhibit the emotional abnormality produced by stress, which is possibly mediated by the dopaminergic mechanisms in the mouse brain.
Essential oils and perillaldehyde from perilla leaves were also found to show an anti-depressant property in mice with CUMS-induced depression. In another study, daily consumption of perillaldehyde (20 mg/kg, oral) demonstrated significant antidepressant-like effects in mice with LPS-induced depression and the authors concluded a potential benefit in inflammation-related depression. Inhaling the same compound (perillaldehyde 0.0965 and 0.965 mg/mouse/day, 9 days) had antidepressant-like properties on a stress-induced, depression-like model in mice during the forced swim test (FST) through the olfactory nervous function.
The oil of PF seeds might have an anti-depressant activity too since a seed oil-rich diet during a forced swim test in adult male rats modulated the fatty acid profiles and brain-derived neurotrophic factor (BDNF) expression in the brain. Moreover, perilla seed oil rich in n-3 fatty acids improved cognitive function in rats by generating new hippocampal neural membrane structures as well as by inducing specific protein expression.
Anti-Inflammatory Activity
Luteolin has been isolated from PFL ethanol extracts and was demonstrated to exert beneficial effects on neuro-inflammatory diseases in a dose-dependent manner (IC50 = 6.9 μM) through suppressing the expression of inducible nitric oxide synthase (iNOS) in BV-2 microglial cells.
The ethanol extract of PFL was identified to display significant anti-inflammatory activity in LPS-induced Raw 264.7 mouse macrophages through the inhibition of the expression of pro-inflammatory cytokines, inhibition of mitogen-activated protein kinase (MAPK) activation, and of nuclear factor-kappa (NF-κB) nuclear translocation in response to LPS. The seed oil from PF showed a great protective effect against reflux esophagitis and this could be attributed to the antisecretory (anticholinergic, antihistaminic), antioxidant, and lipoxygenase inhibitory activities due to the presence of α-Linolenic acid (ALA) (18:3, n-3). Furthermore, RA isolated from PFL could inhibit the release of high mobility group box 1 protein (HMGB1) and down-regulated HMGB1-dependent inflammatory responses in human endothelial cells, HMGB1-mediated hyperpermeability, and leukocyte migration in mice, as well as reduced cecal ligation and puncture (CLP)-induced HMGB1 release and sepsis-related mortality. This could be a potential remediation for various vascular inflammatory diseases, such as sepsis and septic shock, via inhibition of the HMGB1 signaling pathway. Lipophilic triterpene acids from ethanol extracts of red and green PFL were demonstrated to have remarkable anti-inflammatory activity on 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced inflammation in mice (ID50: 0.09–0.3 mg per ear), and on the Epstein–Barr virus early antigen (EBV-EA) activation (91–93% inhibition at 1 × 103 mol ratio/TPA). A recent study in mice showed that PF extract ameliorated inflammatory bowel disease (IBD) via protection of dextran sulfate sodium-induced murine colitis, with NF-κB and signal transduction and activator of transcription 3 (STAT3) as putative targets. A perillaketone-type and alkaloid isolated from aerial parts of perilla showed the remarkable inhibitory effect on pro-inflammatory cytokines (TNF-α and/or IL-6) and inflammatory mediator (NO) in LPS-stimulated RAW264.7 cells, indicating that these compounds might be active components for inflammatory disorders.
Antitumor Effect
A number of in vivo and in vitro studies have reported the potential anticancer activity of PF. Tormentic acid, a lipophilic triterpene acid from ethanol extracts of red and green PFL, remarkably blocked carcinogenenesis in an in vivo, two-stage mouse skin model. Similarly, in an in vivo carcinogenesis model, topical application of perilla-derived fraction (2.0 mg/mouse) led to a significant reduction of 7,12-dimethylbenz(a)anthracene (DMBA)-initiated and TPA-promoted tumorgenesis. This is probably based on two independent effects: inhibition of oxidative DNA injury and inhibition of adhesion molecule, chemokine, and eicosanoid synthesis.
In addition, Lin et al. evaluated the inhibitory effects of PFL and they found that it effectively induces apoptosis-related genes and could inhibit cell proliferation in human hepatoma HepG2 cells. They also observed that the inhibitory effect of PFL was much higher than the same dose of commercially available RA and luteolin compounds.
In another study, the application of ethanol extract of PFL resulted in induced apoptosis through the combinations of death receptor-mediated, mitochondrial, and endoplasmic reticulum stress-induced pathways, and substantially suppressed the cell proliferation via p21-mediated G1 phase arrest in human leukemia HL-60 cells. Isoegomaketone (IK), an essential oil component of PF, was found to be another potential agent possessing anti-cancer activity. IK induces apoptosis through caspase-dependent and caspase-independent pathways in human colon adenocarcinoma DLD-1 cells.
Miscellaneous Effects
In addition to the pharmacological activities described above, different extracts, seed oil, and some individual phenolic compounds of perilla have been found to exhibit other special physiological activities indicating further therapeutic utilizations.
An aqueous extract of PF showed potent anti-HIV-1 activity via inhibition giant cell formation in co-culture of Molt-4 cells with and without HIV-1 infection showing inhibitory activity against HIV-1 reverse transcriptase. A very recent study indicates the importance of PFL extracts as a potential anti-aging agent for skin, as it showed effectiveness against UV-induced dermal matrix damage in vitro and in vivo. The in vitro neuroprotection activity of unsaturated fatty acids of PF seed oil have been reported by Eckert et al. Perilla seed oil might be useful for other complaints too. Deng et al. described in vitro and in vivo anti-asthmatic effects of perilla seed oil in the guinea pig and concluded that the oil may ameliorate lung function in asthma by regulating eicosanoid production and suppressing leukotriene (LT) generation. Zhao et al. supposed a possible anti-ischemic activity of luteolin extracted from PFL, likely through a rebalancing of pro-oxidant/antioxidant status.
In vivo, the protective activity of RA from PFL was demonstrated on LPS-induced liver injury of d-GalN-sensitized mice. The treatments significantly reduced the elevation of plasma aspartate aminotransferase (AST) and alanine transaminase (ALT) levels, as well as anti-TNF and sphincter of Oddi dysfunction (SOD) treatment, compared with controls. In one investigation, the hepatoprotective effects of sucrose-treated perilla leaves, other than untreated leaves, exhibited the best result in vitro and in vivo.
Benfotiamine
Benfotiamine, a lipid-soluble analogue of vitamin B1, is a potent antioxidant that is used as a food supplement for the treatment of diabetic complications. A recent study (U.C. Yadav et al., Free Radic. Biol. Med. 48:1423-1434, 2010) indicates a novel role for benfotiamine in the prevention of bacterial endotoxin, lipopolysaccharide (LPS)-induced cytotoxicity and inflammatory response in murine macrophages. Nevertheless, it remains unclear how benfotiamine mediates anti-inflammatory effects. In this study, we investigated the anti-inflammatory role of benfotiamine in regulating arachidonic acid (AA) pathway-generated inflammatory lipid mediators in RAW264.7 macrophages. Benfotiamine prevented the LPS-induced activation of cPLA2 and release of AA metabolites such as leukotrienes, prostaglandin E2, thromboxane 2 (TXB2), and prostacyclin (PGI2) in macrophages. Further, LPS-induced expression of AA-metabolizing enzymes such as COX-2, LOX-5, TXB synthase, and PGI2 synthase was significantly blocked by benfotiamine. Furthermore, benfotiamine prevented the LPS-induced phosphorylation of ERK1/2 and expression of transcription factors NF-κB and Egr-1. Benfotiamine also prevented the LPS-induced oxidative stress and protein-HNE adduct formation. Most importantly, compared to specific COX-2 and LOX-5 inhibitors, benfotiamine significantly prevented LPS-induced macrophage death and monocyte adhesion to endothelial cells. Thus, our studies indicate that the dual regulation of the COX and LOX pathways in AA metabolism could be a novel mechanism by which benfotiamine exhibits its potential anti-inflammatory response.
Benfotiamine may work by:
· Activating the enzyme transketolase, a necessary part of the pentose phosphate pathway that turns sugars into sources of energy instead of harmful advanced glycation end-products (AGEs)
· Changing the production of specific enzymes (Nos3, PKB/Akt) to increase cell regeneration and reduce cell death
· Altering how energy is used in cells to enhance healthy growth and suppress cancer development
· Decreasing inflammation in brain cells and white blood cells
· Inhibiting the GSK3b gene, which improves brain health
· Inhibiting the NOX4 gene, which improves the breakdown of sugar in muscles.
Apigenin
Apigenin is a flavonoid found in fruits, vegetables, and herbs, including parsley, celery, onions, oranges, chamomile, thyme, and tea. As with other flavonoids, apigenin has been shown to have anti-inflammatory and antiapoptotic effects (eg, in heart cells temporarily deprived of oxygen). In a study on collagen-producing cells called fibroblasts, apigenin reversed the effects of pharmacologically induced senescence by reducing the cells’ pro-inflammatory characteristics. Furthermore, apigenin has been shown to decrease the risk of ultraviolet-induced senescence of collagen-producing cells in the laboratory. When topically applied to aging skin, apigenin was associated with improvements in skin elasticity, fine wrinkles, skin evenness, and moisture content. In mice, the anti-aging properties of apigenin were shown to be attributable in part to modification of gene expression.
Interestingly, the effects of apigenin appear to differ in normal cells versus cancer cells. In normal fibroblasts, apigenin reduces senescent characteristics. In a study on breast cancer cells, apigenin decreased the invasiveness and aggressiveness of the cells. Furthermore, apigenin was shown to increase the sensitivity of glioblastoma cells to apoptosis by inducing cell senescence.
· Antioxidant
· free-radical scavengers
· exhibiting anti-mutagenic
· reduce plasma levels of low-density lipoproteins
· inhibit platelet aggregation
· reduce cell proliferation
· improving detoxification enzymes
· inducing apoptosis
· anti-inflammatory
· anti-amyloidogenic
· neuroprotective
· cognition-enhancing substance with interesting potential in the treatment/prevention of Alzheimer’s disease.
· restoration of the ERK/CREB/BDNF pathway, involved in memory and typically affected in Alzheimer’s disease
· inhibits the activation of cytokines and NO production
· decreased the number of activated microglia of GFAP-IL6 mice both cerebellum and hippocampus by around 30% and 25%, respectively.
· In LPS treated animal studies npigenin attenuated production of pro-inflammatory cytokines interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α). What is more, the authors found that apigenin suppressed inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expression
· has demonstrated to easily penetrate the BBB
· enhances the expression of anti-oxidant enzymes such as GSH-synthase, catalase, and SOD to counteract cellular oxidative and electrophilic stress.
· enhances the expression of phase II enzyme encoding genes by blocking the NADPH oxidase complex and their downstream target inflammatory genes and by increasing the expression of nuclear translocation of Nrf-2
· induce the inhibition of metastasis and angiogenesis by interacting with the signaling molecules in the three major mitogen-activated protein kinase (MAPK) pathways: extracellular-signal-regulated kinase (ERK), c-Jun N-terminal kinases (JNK), and p38 in human cell culture models
· strongly decreased levels of interleukin 6 (IL-6), which in general acts as both a pro-inflammatory cytokine and an anti-inflammatory myokine, in lipopolysaccharide (LPS)-activated mouse macrophages
· suppressing cluster of differentiation 40 (CD40), tumor necrosis factor (TNF-α), and IL-6 production via inhibition of interferon gamma (IFN-γ)-induced phosphorylation of signal transducers and activators of transcription 1 (STAT1) in murine microglia
Healing Properties of Apigenin: An Overview
In recent years interest in apigenin as a beneficial and health-promoting agent has grown.
Recently Kashyap et al. summarized the multiple therapeutic functions of apigenin by in vitro and in vivo systems. The different mechanisms underlying the potential therapeutic action of apigenin were explored, including cell cycle arrest, apoptosis, anti-inflammatory, and antioxidant function. Apigenin induces cell cycle arrest at different proliferation stages including G1/S-phase or G2/M phase by modulating the expression of different CDKs and other genes. It is known that apigenin can regulate intrinsic apoptotic pathways, changing mitochondrial membrane potential and causing the release of cytochrome C in the cytoplasm, which subsequently forms APFA, activates caspase 3, and turns on apoptosis. Otherwise, apigenin regulated extrinsic apoptotic pathways by involving caspase-8 activation. In cancer cells, apigenin activates apoptosis by modulating Bcl-2, Bax, STAT-3 and Akt proteins expression.
Apigenin promotes different anti-inflammatory pathways, including p38/MAPK and PI3K/Akt, as well as prevent the IKB degradation and nuclear translocation of the NF-κB, and reduce COX-2 activity. In human cell cultures, apigenin has demonstrated the ability to inactivate nuclear factor kappa-light-chain-enhancer or to activate B cells (NF-κB), mediated by suppression of LPS-induced phosphorylation of the p65 subunit.
It is believed that apigenin decreases the expression of adhesion molecules, which is a defensive strategy against oxidative stress, such as free-radical scavenging.
Apigenin enhances the expression of anti-oxidant enzymes such as GSH-synthase, catalase, and SOD to counteract cellular oxidative and electrophilic stress. It also enhances the expression of phase II enzyme encoding genes by blocking the NADPH oxidase complex and their downstream target inflammatory genes and by increasing the expression of nuclear translocation of Nrf-2.
Apigenin is also reported to induce the inhibition of metastasis and angiogenesis by interacting with the signaling molecules in the three major mitogen-activated protein kinase (MAPK) pathways: extracellular-signal-regulated kinase (ERK), c-Jun N-terminal kinases (JNK), and p38 in human cell culture models. It is known that apigenin strongly decreased levels of interleukin 6 (IL-6), which in general acts as both a pro-inflammatory cytokine and an anti-inflammatory myokine, in lipopolysaccharide (LPS)-activated mouse macrophages. This flavonoid is also known for suppressing cluster of differentiation 40 (CD40), tumor necrosis factor (TNF-α), and IL-6 production via inhibition of interferon gamma (IFN-γ)-induced phosphorylation of signal transducers and activators of transcription 1 (STAT1) in murine microglia.
Furthermore, after absorption into the digestive tract, apigenin is able to reach the brain through the circulatory system, where it can cross the blood‒brain barrier before exerting its affinity with the GABAA-receptor and acting on the CNS, despite its action at the level of improving the clinical use of benzodiazepines is not clear. Sloley et al. reported the in vitro inhibition of rat-brain monoamine oxidases (MAOs) by apigenin, a family of enzymes of flavin-containing amine oxidoreductases, present in human neurons and astroglia. Unregulated MAO activity could be responsible for a number of psychiatric and neurological disorders and their inhibitors, like apigenin, work as antidepressant and antianxiety agents, as well as to treat Alzheimer’s and Parkinson’s disease.
Baicalin
Traditional Chinese medicine is one of the world's oldest documented medical systems based on herbal remedies. Scutellaria baicalensis, the most widely used herb, has been in use for thousands of years.
Baicalin and its aglycon baicalein are the principal components found among other flavonoid derivatives in the roots of Scutellaria baicalensis Georgi.
Abundant scientific evidence shows that the neuronal protective effects of baicalin and baicalein are related to anti-oxidant, anti-apoptotic, anti-inflammatory and anti-excitotoxicity effects, protection of the mitochondria, promotion of neuronal protective factors expression and adult neurogenesis and other factors.
· Both baicalin and baicalein as potent antioxidants ameliorated various inflammatory disorders.
· These flavonoids prevented inflammatory disorders through NF-κB inactivation, cytokines and ROS down-regulation and cell cycle arrest.
The flavonoids, baicalin (5,6-dihydroxy-2-phenyl-4H-1-benzopyran-4-one-7-O-d-β-glucuronic acid) 1 and its aglycone, baicalein 2 are found in edible medicinal plants, Scutellaria baicalensis Georgi and Oroxylum indicum (L.) Kurz in abundant quantities. The antioxidant and anti-inflammatory effects of these flavonoids have been demonstrated in various disease models, including diabetes, cardiovascular diseases, inflammatory bowel diseases, gout and rheumatoid arthritis, asthma, neurodegenerative-, liver- and kidney diseases, encephalomyelitis, and carcinogenesis.
These flavonoids have almost no toxicity to human normal epithelial, peripheral and myeloid cells. Their antioxidant and anti-inflammatory activities are largely due to their abilities to scavenge the reactive oxygen species (ROS) and improvement of antioxidant status by attenuating the activity of NF-κB and suppressing the expression of several inflammatory cytokines and chemokines including monocyte chemotactic protein-1 (MCP-1), nitric oxide synthase, cyclooxygenases, lipoxygenases, cellular adhesion molecules, tumor necrosis factor and interleukins. we summarize the antioxidant and anti-inflammatory effects of baicalin and baicalein with molecular mechanisms for their chemopreventive and chemotherapeutic applications in the treatment of inflammatory-related diseases.
The neuroprotective effects of baicalein and baicalin.
Vitamin A
The term vitamin A indicates both retinol and its analogues, called retinoids, of which at least 1500 different types are known, both natural and synthetic in origin. Carotenoids that contain at least one unsubstituted β-ionone ring (such as beta-carotene) are also considered to be precursors of vitamin A. According to available data, vitamin A and its metabolites are able to regulate both innate and adaptive arms of the immune response, by increasing IL-2 secretion, and to modulate proliferation, differentiation and cytokine signaling as well as production, all in T, B and antigen-presenting cells. Vitamin A metabolites also modulate more specific functional aspects of the immune response, such as the TH1–TH2-cell balance and the differentiation of TReg cells and TH17 cells. In particular, depending on the physical and biochemical composition of the microenvironment, vitamin A, in the presence of different concentrations of cytokines and transcription factors, stimulates TH 0 cells to assume a TH-2 phenotype, while inhibiting TH-1 subtypes. Furthermore, in the presence of an adequate concentration of transforming growth factor-β (TGFβ), retinoic acid promotes TReg-cell differentiation in peripheral tissues, whereas it inhibits the progression of lymphocytes from TH0 to pro-inflammatory TH-17. Retinoic acid displays gut imprinting ability on T and B cells and induces the differentiation of B cells to IgA-producing plasma-cells. A severe defect in intestinal immune responses with enhanced mortality is detectable in patients with vitamin A deficiency. These subjects suffer from gastrointestinal and respiratory infections. On the other hand, vitamin A administration is associated with a significant reduction in diarrhea and mortality in children with HIV infection or malnourishment. Moreover, vitamin A is able to stimulate the intracellular type I IFN system, which exerts antiviral activities.
Vitamin D3
1,25(OH)2VD3 acts on T cells down-regulating T-helper-1 (TH1)-cell cytokines, particularly IFNγ, and stimulating TH2-cell responses.
This event is induced by the decrease of IFNγ release and by the enhancement of IL-4 production. Furthermore, 1,25(OH)2 VD3 regulates effector T-cell differentiation by modulating antigen-presenting DCs, which decrease their synthesis of IL-12, a cytokine that promotes TH1-cell responses. 1,25(OH)2VD3 also exerts an important effect on the activity of the immune system, by counteracting TH17-cell responses and the differentiation of naive TH-0 cells into a TH-17 type, as this micronutrient is able, in part, to down-regulate several pro-inflammatory cytokines, including IL-6, IL-17 and IL-23 production, and promotes the reciprocal differentiation and proliferation of forkhead box protein 3 (FOXP3) + regulatory T (TReg) cells.
Furthermore, 1,25(OH)2VD3 decreases B-cell expansion, plasma-cell development and IgG secretion, probably by modulating the activities of antigen presenting-cells (APC) and by a means of a direct action on B cells.
In addition, 1,25(OH)2VD3 decreases the synthesis of IL-12 and simultaneously increases the production of IL-10 by DCs. Therefore, the TH1-cell response is shifted to a T regulatory type 1 (TR1) cell response. The results emerging from these experimental studies in patients with rheumatoid arthritis have provided the rationale for the use of 1,25(OH)2VD3 in association with other drugs, including the anti-IL-6 receptor monoclonal antibody (Tocilizumab), in the treatment of patients suffering from rheumatoid arthritis of various severity.
Interestingly, the best response (assessed by means of Diseases Activity Scores) to the therapy was obtained in patients with sufficient serum 25(OH)D levels (≥ 30 ng/mL) when tocilizumab was initiated, in comparison with patients with lower serum 25(OH)D levels (< 30 ng/mL). 1,25(OH)2VD3 primarily exhibits inhibitory activities on the adaptive immune response.
However, in some circumstances, it may enhance the release of IL-1 by both monocytes and macrophages. Vitamin D is able to stimulate the intracellular type I IFN system, which exerts antiviral activities.
Riboflavin
Riboflavin (RF) was first documented in 1879 by Blythto as a yellow pigment found in milk. RF, chemically, is 7, 8-dimethyl-10-ribityl-isoalloxazine which consists of a flavin isoalloxazine ring bound to a sugar side chain, Ribitol. RF is also known as an essential vitamin B2, a water-soluble vitamin, is heat stable. Cooking does not lower levels of RF, however exposure to light could destroy it. RF can be found in a wide variety of foods and natural sources, especially milk, organ meats—mostly in calf liver, egg, fish, nuts, certain fruits and legumes, wild rice, mushrooms, dark green leafy vegetables, yeast, beer, cheese and dietary products.
RF is poorly stored by vertebrates because of its limited absorption in humans. Therefore, orally supplied RF by a healthy diet is required to avoid ariboflavinosis which causes cheilitis, sore tongue, and a scaly rash on the scrotum or vulva.
RF causes no known toxicity, since at higher intakes it is excreted in the urine and not stored. RF is found in different concentrations in various human body fluids and organs.
Recent analytical procedures developed for detection of RF in biological samples were described in several reviews. RF is phosphorylated intracellular to flavin mononucleotide (FMN) and further metabolized to flavin adenine dinucleotide (FAD). Both FMN and FAD play a key role as cofactors in energy metabolism and are required for co-enzyme function in numerous oxidation and reduction reactions in all aerobic forms of life. RF has been used in dietary supplements and for inflammatory disease treatments such as angulus infectious, cheilitis, glossitis , sepsis, cataracts and migraine headaches.
Antioxidant Properties
Oxidative stress plays a crucial functional role in the pathogenesis of various human disease states including ischemia-reperfusion injury, diabetes and angina pectoris. Oxidative stress is a key effect of aging and degenerative diseases along with aging. The authors have demonstrated that supplementation of RF significantly extended the lifetime and strengthened the reproduction of fruit flies via enhancing the activity of antioxidant enzymes.
RF also activates the synthesis of a normal extracellular matrix and reduces reactive oxygen species (ROS) levels in keratoconus. The evidence of the effect of RF and its roles for reactive oxygen species in various symptoms and diseases. RF was used for its potent antioxidant and anti-inflammatory effects in the ischaemic liver, protecting hepatic parenchymal cells against I/R injury. Other antioxidant enzymes concentrations—like superoxide dismutase (SOD), catalase and glutathione peroxidase—are also influenced by RF concentration. RF plays an important role for the antioxidant status inside cell systems as well as being part of the glutathione reductase (GR) and xanthine oxidase system. RF in the form of FAD is necessary for GR enzyme to convert oxidized glutathione (GSSG) to the reduced glutathione (GSH). It then functions as an endogenous antioxidant in different cells.
Antioxidant Properties Oxidative stress plays a crucial functional role in the pathogenesis of various human disease states including ischemia-reperfusion injury, diabetes and angina pectoris. Oxidative stress is a key effect of aging and degenerative diseases along with aging. The authors have demonstrated that supplementation of RF significantly extended the lifetime and strengthened the reproduction of fruit flies via enhancing the activity of antioxidant enzymes.
RF also activates the synthesis of a normal extracellular matrix and reduces reactive oxygen species (ROS) levels in keratoconus. RF was used for its potent antioxidant and anti-inflammatory effects in the ischaemic liver, protecting hepatic parenchymal cells against I/R injury.
Other antioxidant enzymes concentrations—like superoxide dismutase (SOD), catalase and glutathione peroxidase—are also influenced by RF concentration. RF plays an important role for the antioxidant status inside cell systems as well as being part of the glutathione reductase (GR) and xanthine oxidase system. RF in the form of FAD is necessary for GR enzyme to convert oxidized glutathione (GSSG) to the reduced glutathione (GSH). It then functions as an endogenous antioxidant in different cells.
Reperfusion Oxidative Injury
Reperfusion oxidative injuries refer to the tissue damages which occur after ischemia when free radicals and inflammatory cytokines are increased. RF can attenuate oxidative injuries through its ability to scavenge free radicals and therefore decrease re-oxygenation injuries. New evidence suggests that ROS have a crucial role in the pathogenesis of ischemia/reperfusion injury. Studies in various cell cultures and animal species have shown a variety of protective actions of RF in many organs, e.g., the neuroprotective effect of cerebral ischemia. The authors showed that pre-treatment of the SH-SY5Y cells with RF before oxygen glucose deprivation (OGD) experiments significantly reduced the OGD-induced lactate dehydrogenase (LDH) release and significantly increased cell viability. The cell viability and LDH secretion were quantified in the SH-SY5Y cell line and cortical neuron cultures using the cell counting Kit-8 and the LDH quantification kit compared to that treated-OGD group. In vivo study showed a significant neuroprotective activity via the modulation of the miR-203/c-Jun signalling pathway. RF can significantly protect against oxidant-mediated inflammatory injury in the lungs of Long-Evans rats caused by cobra venom factor or IgG immune complexes, or ischemia-perfusion. RF has also been reported to have a protective role in focal ischemia with decreasing brain injury and oedema formation in rats. RF also has cardio-protective effects in isolated rabbit cardiomyocytes, reducing elevated ferrylmyoglobulin induced by cardiac re-oxygenation damage. This effect is mediated by Flavin reductase.
Immune System
RF activates phagocytic activity of neutrophils and macrophages, and stimulates the multiplication of neutrophils and monocytes. It has also been shown that RF is important for the survival of macrophage RAW 264.7 cells. The reduction in RF concentration resulted in a decreased rate of cell proliferation. A combined supplementation—consisting of RF, delta-tocotrienol and quercetin—improved the inhibition of serum tumor necrosis factor alpha (TNF-α) and nitric oxide (NO) levels in a chicken model.
However, RF administration affects neutrophil migration, inhibiting the infiltration and accumulation of activated granulocytes into peripheral sites, which may lead to a decreased inflammatory influx and, thereby, a decrease in inflammatory symptoms.
RF is a potential substance for use in virus inactivation, or as an adjuvant in chemo radiotherapy for cancer treatment because of its toxicological and photosensitizing attributes. RF suppressed T-cells infiltration and donor-reactive alloantibody formation during the early period after allotransplantation. The pro-inflammatory transcription nuclear factor kappa B (NF-κB) is normally activated by degradation of inhibitory kappa B (IκB).When this occurs, NF-κB translocates to the nucleus and binds to specific promoter regions of genes encoding pro-inflammatory proteins. Proteasomes are key regulators of lipopolysaccharide (LPS)-stimulated inflammatory signalling pathways. RF, as proteasome inhibitor, possibly down-regulates the NF-κB activation initiated by ROS, which are the potent activators of a plethora of general pro-inflammatory cytokines such as interleukin-6 (IL-6), TNF-α, etc. Therefore, ultimately, as proteasome inhibitor RF suppresses the production of TNF-α and NO, and exerts anti-inflammatory effects by inhibiting NF-κB, activation. As was recently reported, RF may protect against multitude of age-associated diseases by inhibition levels of secretion of TNF-α, NO production, activation of NF-κB, and degradation. In recent years, there has been much interest in the anti-nociceptive and anti-inflammatory effects of RF. RF helps in reducing inflammatory nociceptive pain.
Several animal models have been used to study the possible role in anti-nociceptive and anti-inflammatory effects of RF. It has been indicated that RF could inhibit nociceptive responses induced by a number of inflammatory agents in a variety of structures. For example, RF inhibited the formalin-induced hind paw oedema. Moreover, RF can improve the anti-nociceptive effect when combined with low-dose morphine in a formalin test model, as well as in a zymosan-induced peritonitis model. The anti-inflammatory studies of RF on the zymosan-induced peritonitis model showed that RF effects were dependent on the time of administration and dose, as well as strain-specific differences in mice.
Mechanism of Antioxidant Protection
RF has an antioxidant function that can destroy ROS. The powerful antioxidant activity is derived from its role as precursor to FMN and FAD as important coenzymes required by a number of enzymes involved in oxidative metabolism, particularly glutathione oxidase. On the other hand, GR—which is a free radical scavenger—also needs FAD as a cofactor. The protective role of RF against various diseases has been described previously. Several studies showed that RF is effective to reduce ROS in various diseases including anti-aging and it also helps to ameliorate the toxic effect of drugs in various treatments. Many studies investigated the antioxidant effect of some vitamins such as vitamin E, vitamin C, carotenoids and RF. RF effective mechanism has not been completely investigated. It acts as a coenzyme for redox enzymes in FAD and FMN forms and can have a potential effect on oxidative stress reduction as an antioxidant by prevention of lipid peroxidation and by attenuation of reperfusion oxidative injury. GR requires RF for its activity to convert GSSG to the GSH. convert GSSG to the GSH. FAD transports hydrogen from NADPH to GSSG to convert it into the GSH form. GSH acts intracellular as an endogenous antioxidant and deactivates ROS. Glutathione deactivates peroxides such as hydroperoxides by the action of GPx from GSH to lipid peroxide and produce GSSG and alcohol. Therefore, RF deficiency leads to an increase in lipid peroxidation.
Bio Peptide Regulators
The naturally active peptide bioregulator consists of a mixture of reduced molecular peptides with weight up to 10000 Da, isolated from the CNS of young animals.
The Bio Peptides Regulators have tissue specific action on cells of the tissues from which they were isolated according to experimental studies. Regulation of metabolic processes by peptides boosts brain safety margins. Processes of adaptation in organisms become favorable in severe conditions, and they have adverse oxidative properties, balancing peroxide oxidation processes in the brain cortex. It is probable that administration of CNS Bio Peptide Regulators could be efficient and beneficial in the restoration of the function of the central nervous system and its disorders of different origins.
Diseases of the central nervous system lead to the invalidation of patients and social adaptation disorder, therefore, its treatment is of definite importance.
The various kinds of action used in traditional therapeutic agents to carry out treatment of patients with diseases of the central nervous system of pathogenetic mechanisms include influence on metabolism and integrative functions of the brain and reduction of psychopathological signs. They also involve normalization of cerebral and systematic circulation, correcting changes of bioelectric activity of the brain, inhibition and prevention of adhesive process, influence on liquor dynamic disorders and correcting immunopathological reactions.
It is effective for complex recovering of central nervous system functions after diseases of various genesis, at the pathological states leading to violation of brain functions, at influence of external environment extreme factors, malnutrition, and also at aging.
Recommended for…
Help:
· promote healthy cytokine and mast cell activities,
· transition out of phase I Cell Danger Response or Survival Mode
· normalize of brain activity while atherosclerosis,
· during rehabilitation after stroke and craniocereberal trauma,
· decrease in memory and violation of attention concentration,
· lowering symptoms of Alzheimer and Parkinsons diseases,
· lowering symptoms of disseminated sclerosis,
· lowering symptoms of syndrome of chronic fatigue,
· lowering symptoms of depression,
· lowering symptoms of neuralgia and neuritis,
· lowering symptoms of emotional instability, especially of elderly people.
*Your results may vary from those listed above.
*These statements have not been evaluated by the Food and Drug Administration.
*This product is not intended to diagnose, treat, cure or prevent any disease.
Since we do not know everything about your medical history and medications, please consult with your health care practitioner before implementing any new protocols and supplements. Do not construe any information listed on this site as a substitute for actual medical advice. The info you receive from us is not intended to replace medical advice by your doctor. Forrest Health, Inc. does not dispense medical advice, prescribe, or diagnose illness. We offer nutritional programs and supplements that support your health. The views and nutritional advice expressed by Natural ReGenesis. are not intended to be a substitute for conventional medical service. If you have a medical condition, see your physician of choice.